
- Home
- Products
- Baghouse Dust Collectors
Additive dosing
Additive dosing
The addition of the adsorbent takes place on the pneumatic way. The hydrated lime is taken out of a silo through a slide gate, a rotary valve and a screw conveyor. In an intermediate vessel the adsorbent will be weighted and blown into the ductwork. The dosing quantity can be adjusted by a frequency drive so that the necessary amount of adsorbent can be adapted.
Additive Dosing Systems can be placed under a Silo for smaller amounts of additive consumption. Additives can be Hydrated Lime (separation of HCL or HF), Activated Carbon (separation of dioxines) or a mixture of both (for small amount of dioxines).
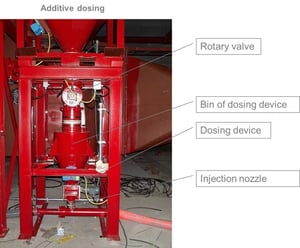
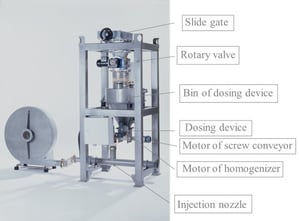
Different Additives
There are basically three different uses of additive:
- Chemical reaction / chemical reaction. - This mostly eliminates acidic pollutants, such as sulphurous acid (SO2), hydrochloric acid (HCl) or hydrofluoric acid (HF). Almost exclusively lime-based products are used here, such as hydrated Lime or Sodium Bicarbonate
- Adsorption for reducing of volatile hydrocarbons, dioxins and mercury - Products such as activated carbon or hearth furnace coke are used. Disadvantage here is the explosiveness of these substances. Mixture of carbohydrate and activated carbon may be employed, or a zeolite material, e.g. Minsorb. These additives bind through a very porous structure (about 1000m² / g) the above mentioned substances. There is no chemical reaction but a purely physical binding / adsorption.
- Binder used for oily exhaust air. - Construction Lime or Stone Meal. Gives a protective layer for the filter bags made of needle felt or cartridges.


Sorption of acidic gases (often from thermal furnace processes)
All acids are chemically converted to salts.
These salts can be cleaned and disposed of in the filter system as a normal dust filtration process.
- Ca(OH)2 + 2HCl → CaCl2 + 2H2O - Calcium Chloride
- Ca(OH)2 + SO2 → CaSO3 + H2O - Calcium Sulphite
- CaSO3 · H2O + ½O2 + H2O → CaSO4 · 2H2O - further reaction to Calcium Sulphate dihydrate (gypsum)
- Ca(OH)2 + 2HF → CaF2 +2H2O - Calcium Fluoride
- Ca(OH)2 + CO2 → CaCO3 + H2O - Calcium Carbonate
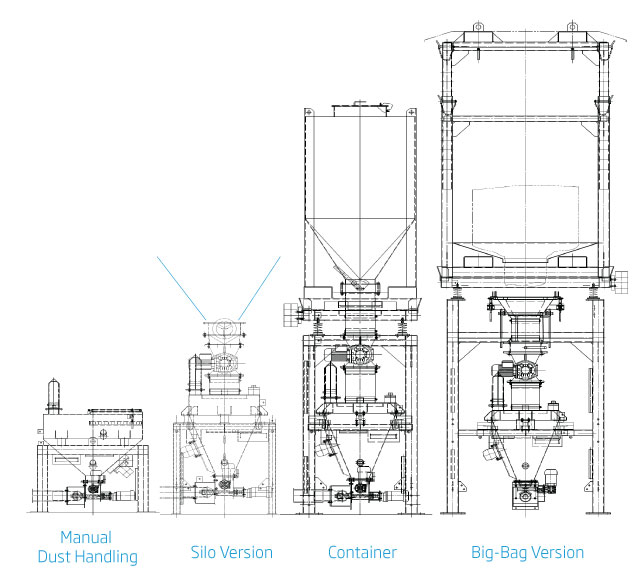
Additive Dosing System Versions
Contact us to create your custom solution
Call us at: 704-859-2723 or fill out the form below.